One of the basic chemical combinations P means power in chemistry and H means hydrogen ions thus, pH means power of hydrogen ions. The acidity and alkalinity of a substance are measured using a scale of numbers which ranges from 0 to 14 called the pH scale. It measures the concentration of hydrogen ions in a solution. Remember, the Arrhenius definition of an acid that says that an acid is a compound which when dissolved in water produces hydrogen ion as the only positive ion while according to Bronsted-lowry, an acid is a proton donor and a base is a proton acceptor. Recall that a proton is a hydrogen ion.
HCl → H+ + Cl–
H2SO4 → 2H+ + S −
HYDROGEN ION CONCENTRATION
To understand this, we have to critically look at the dissociation of water. Pure water is a neutral solution. It ionizes very slightly to give equal concentrations of hydrogen ions and hydroxide ions. This is confirmed by conductivity measurements which shows that at 25oC, the concentration of hydrogen ions [H+] and the concentration of the hydroxide ions [OH–] are both equal to 10-7 moldm-3 respectively.
H2O(l) H+(aq) + OH—(aq)
1mol 1mol 1mol
From the above equation, it shows that 1mol of water dissociates into 1mol of hydrogen ions and 1 mol of hydroxide ions.
[H+] = [OH–] =10-7moldm-3
THE IONIC PRODUCT OF WATER KW
The ionic product of water is the product of the concentration of H+ and OH–
KW = [H+] [OH–]
=10-7moldm-3 x 10-7moldm-3
=10-14mol2dm-6.
The ionic product of water, KW is kept constant under all circumstances at 25oC. This means that in all neutral solutions, the concentration of both hydrogen ions and hydroxide ions would be 10-7moldm-3 respectively. If an acid is added to an aqueous solution, the hydrogen ion concentration increases to above 10-7 moldm-3. In order to maintain the ionic product of water, KW at 10-14 mol2dm-6, the hydroxide ions would decrease proportionally. For instance, if concentration of the [H+] increases from 10-7 moldm-3 to 10-4moldm-3, the concentration of [OH–] will decrease from 10-7moldm-3 to 10-10moldm-3. The solution becomes more acidic since the concentration of H+ ions is more than the concentration of OH– ions. A solution becomes more alkaline if the concentration of hydroxide ions is more than the concentration of hydrogen ions.
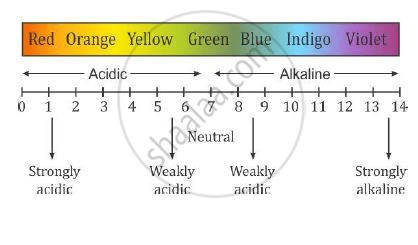
pH SCALE
pH scale ranges from 0 to 14 . As the value of pH decreases, the acidity of the solution increases and as the value of pH increases, the acidity decreases while the alkalinity increases. The pH of a neutral solution is 7.
This means that a value of less than 7 on the scale is acidic while a value greater than 7 on the scale is alkaline. Note that the pH measures strengths of acids and alkalis and NOT their concentrations. The lower the pH value on the scale, the stronger the acid hence, an acid with a pH value of 1 is stronger than an acid with a pH value of 4. Always remember that as acidity decreases, alkalinity increases and vice versa. When acidity increases, it means that H+ increases in the solution and when alkalinity increases, it means that OH– increases in the solution.
pH OF A SOLUTION
There are three definitions:
- pH of a solution is a measure of the hydrogen ions concentration in the solution
- pH of a solution is a measure of the acidity or alkalinity of the solution
- According to Sorensen, pH of a solution is defined as the negative logarithm to base 10 of the hydrogen ion concentration. Mathematically,
pH = – log10 [H+]
Note that:
- The negative sign is introduced to make the pH values positive in most cases.
- The logarithms used is to base 10 (not to the base of e),so make sure that when doing calculations, you press the log or lg button on your calculators NOT the ln button.
- We can use this equation to convert [H+] to pH or pH to [H+].
Calculations involving the pH or POH are done using various formulae. The particular one to be used depends on the question. The formulae are as illustrated below:
- pH = -log10 [H+]
- [H+][OH–] = 10-14
- pOH = 14 – pH
- To calculate the [H+] from pH, the antilog of the pH will be used.
CALCULATING pH FROM [H+]
1. Calculate the pH of a solution whose H+ ion concentration is 5.32 x 10-4 moldm-3.
pH = -log10[H+]
- – log10[5.32×10-4]
- 3.3
- Calculate the pH of 0.005moldm-3 H2SO4 The first thing to do is to dissociate the acid H2SO4 → 2H+ + S 42−
1mol 2mols 1mol
0.005 2×0.005
=0.0100moldm-3
pH = -log10[0.0100]
- – log10[1 x 10-2]
- 2log1010
pH = 2.
CALCULATING [H+] FROM pH
To calculate H+ concentration from pH, antilog of the pH is determined.
1. Calculate the hydrogen ion concentration of a solution whose pH is 10.5.
pH = -log10 [H+]
[H+] = 10-pH
- 10-10.5
- 3.16 x 10-11moldm-3
- A glass of orange juice is found to have a pOH of 11.40. Calculate the concentration of the hydrogen ions in the juice
pH + pOH = 14
pH = 14 – pOH
=14 – 11.40
=2.60
pH = – log10[H+]
2.60 = – log10[H+]
-2.60 = log [H+]
Antilog of – 2.60 = 2.51 x 10-3moldm-3
3. A solution has a pH of 4. What is the [H3O+] concentration?
Note that H+ and [H3O+] can be used interchangeably.
pH = -log10[H3O+]
- = -log10[H3O+] -4 = log10[H3O+]
Antilog of -4 = 1.00 x 10-4moldm-3
- What is the hydrogen ion concentration of 0.100moldm-3 NaOH solution? We first of all, dissociate NaOH.
NaOH → Na+ + OH–
6
1mol 1mol 1mol
0.100mol 0.100mol
[H+][OH–] = 10-14
H+ = 1 x 10-14
[OH–]
- 1 x 10-14
1 x 10-1
[H+] = 1x 10-13moldm-3